Background
At the outset of the coronavirus infectious disease 2019 (COVID-19) pandemic, hematological malignancy was recognized as a risk factor for severe COVID-19 disease, particularly patients with lymphoma, presenting a unique challenge for optimal management of indolent non-Hodgkin lymphoma (iNHL). To this end, professional societies including ASH favoured less immunosuppressive treatment regimens and omission of rituximab maintenance therapy. However, the implications of these recommendations on the care delivery and outcomes of patients with iNHL are unclear. Accordingly, we examined treatment selection, healthcare utilization, and COVID-19 outcomes of patients with iNHL receiving front line systemic treatment during pre-pandemic vs. pandemic period.
Methods
We performed a retrospective cohort study using administrative databases in Ontario, Canada, comparing outcomes in patients with iNHL who initiated first-line (1L) treatment with rituximab (R) monotherapy, or with bendamustine (B) or cyclophosphamide, vincristine, prednisone (CVP) from Jan 1, 2015 - Dec 31, 2018 (pre-pandemic cohort) and from Sept 1, 2019 - Aug 1, 2020 (pandemic cohort), with end of follow-up Mar, 31 2022. The primary outcome was comparison of treatment pattern before and during the COVID-19 pandemic which includes the following: 1L regimen received (R-CVP vs. B-R vs. R monotherapy), number of cycles received, dose delays, use of rituximab maintenance, and time-to-completion of full rituximab maintenance course (funded in Ontario every 3 months for 8 doses). Secondary outcomes were death, toxicities, healthcare utilization (emergency department [ED] visit, hospitalization, and ICU admission), and COVID-19 outcomes (SARS-CoV-2 PCR-confirmed infection, ED visit, hospitalization, and death). Adjusted odds ratios (aOR) and hazard ratios (aHR) with 95% confidence intervals (CI) from logistic regression and cause-specific proportional hazards models, respectively, were used to estimate associations between covariates (age, sex, comorbidity burden as measured by Aggregated Disease groups) and outcomes.
Results
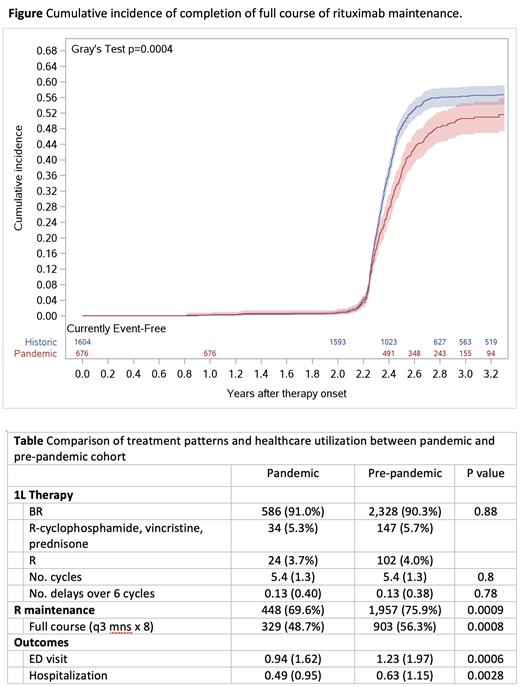
We identified 4,143 patients (1,079 pandemic, 3,064 pre-pandemic), median age 69 (interquartile range 60-76) years, 44% female. In both pre- and pandemic periods, B-R was the most frequent prescribed regimen, with no difference in number of cycles given or in treatment delays (Table). During the pandemic, fewer patients received rituximab maintenance (69.6% vs 75.9%; aOR 0.72, 95% CI 0.60-0.88, p=0.0012). Older age was associated with lower odds of rituximab maintenance initiation (age 75+ vs. age < 60 aOR 0.44, 95% CI 0.35-0.56, p<0.0001). Moreover, patients who initiated maintenance during the pandemic period were less likely to complete the full maintenance course (48.7% vs 56.3%) (aHR 0.81, 95% confidence interval [CI] 0.71-0.92, p=0.0010) (Table) (Figure).
Patients treated during the pandemic had less healthcare utilization (ED visit aHR 0.77, 95% CI 0.68, 0.88, p<0.0001; hospitalization aHR 0.81, 95% CI 0.70-0.94, p=0.0067) and treatment-related complications (infection aHR 0.69, 95% CI 0.57-0.82, p<0.0001; febrile neutropenia aHR 0.66, 95% CI 0.47-0.94, p=0.020), with no difference in death (aHR 0.79, 95% CI 0.58-1.08, p=0.14, Table).
For COVID-19 outcomes, there were 131 SARS-CoV-2 infections from the beginning of the pandemic until December 31, 2021; with follow-up until March 31, 2022, there were 30 ED visits, 56 hospital admissions, and 23 ICU admissions, and 45 deaths related to COVID-19. Rituximab use (first dose to 1-year post-last dose) was associated with higher risk of COVID-19 infection (aHR 1.56, 95% CI 1.09-2.24, p=0.015) and COVID-19 complications (ED visit aHR 4.28, 95% CI 1.79-10.26, p=0.001; hospitalization/death 1.81, 95% CI 1.11-2.93, p=0.016). Primary vaccination series was associated with lower risk of infection and severe outcomes (infection aHR 0.52, 95% CI 0.28-0.97, p=0.04; hospitalization/death aHR 0.36, 95% CI 0.14-0.94, p=0.036; death aHR 0.26, 95% CI 0.07-0.89, p=0.033).
Conclusion
During the pandemic, B-R remained the preferred regimen for iNHL treatment, while rituximab maintenance use was less. Despite similar 1L regimen use, healthcare utilization and infectious complications were less in the pandemic cohort. Rituximab use was associated with nearly 2-fold risk of COVID-19 hospitalization/death.
Disclosures
Prica:Kite-Gilead: Honoraria; Astra-Zeneca: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal